| |
The Mauritius Accreditation Service (MAURITAS)
MAURITAS is the sole national accreditation body and has been established under the Mauritius Accreditation Service Act 1998 as a department within the Ministry responsible for the subject of Industry to provide a national, unified service for the accreditation of calibration and testing laboratories, inspection bodies and certification bodies.
MAURITAS is responsible for the accreditation of:
-
Certification bodies to ISO/IEC 17021-1 - Conformity assessment - Requirements for bodies providing audit and certification of management systems;
-
Product Certification bodies to ISO/IEC 17065 - Conformity assessment - Requirements for bodies certifying products, processes and services;
-
Laboratories (testing and calibration) to ISO/IEC 17025 - General requirements for the competence of testing and calibration laboratories;
-
Medical laboratories to ISO 15189 - Medical laboratories - Particular requirements for quality and competence;
-
Inspection bodies to ISO/IEC 17020 - Requirements for the operation of various types of bodies performing inspection.
MAURITAS certificates are a formal recognition that an organization is technically competent to perform specific tasks.
MAURITAS
RECOGNISED BY ILAC MRA and IAF MLA
MAURITAS was admitted as a signatory member to the ILAC MRA
and the IAF MLA on 04 October 2018 and 17 October 2018
respectively. Further to the 2018 Joint IAF-ILAC Annual
Meetings on 22-31 October, the certificates of recognition
were presented to the Director of MAURITAS on 30 October 2018.
The IAF MLA scope of MAURITAS has been extended to include the
Sub-scope: Level 4: ISO/TS 22003; Sub-scope: Level 5: ISO
22000 for Food Safety Management System since 05 November
2020.
 |
 |
Latest News
- Grants New Accreditation to the Sir Edgar Laurent
TB Laboratory, Central Health Laboratory, Ministry of Health and
Wellness
MAURITAS has granted accreditation to the Sir Edgar Laurent TB Laboratory, which is part of the Microbiology department of the Central Health Laboratory, Ministry of Health and Wellness, on 23 October 2025. This followed an on-site assessment conducted by a team of both local and foreign assessors.
The laboratory is accredited for testing in the medical field, in accordance with the international standard ISO 15189, for 2 parameters.
The Certificate and Schedule of Accreditation for the laboratory are available here. - MAURITAS Grants New Accreditation to the SGS (Mauritius)
Ltd – Testing Laboratories
MAURITAS has granted accreditation to the SGS (Mauritius) Ltd – Testing Laboratories, on 05 September 2025, in accordance with the international standard ISO/IEC 17025.
This followed a rigorous assessment process, including a comprehensive on-site evaluation conducted by a team of MAURITAS assessors.
The laboratory has been accredited for testing in the chemical, biological, and textiles & garments fields, covering a total of 350 parameters.
The Certificate and Schedule of Accreditation for the laboratory are available here. - Publication of new Information Security Management Standard - ISO/IEC 27006-1:2024 and IAF MD 29:2024
- Publication of new Food Safety Standards-ISO 22003-1:2022 and IAF MD 27:2023
Events Gallery
World Accreditation
Day (WAD) 2024 Celebrations
Trainings
carried out by MAURITAS
ILAC/IAF Mutual Recognition Arrangement
Our Vision
- Achieving International Recognition
Our Mission
- To provide a national, impartial and transparent mechanism for the accreditation of laboratories, certification and inspection bodies.
- To achieve international recognition by becoming signatory to International Laboratory Accreditation Cooperation Mutual Recognition Arrangements (ILAC MRA) and International Accreditation Forum Multilateral Recognition Arrangement (IAF MLA).
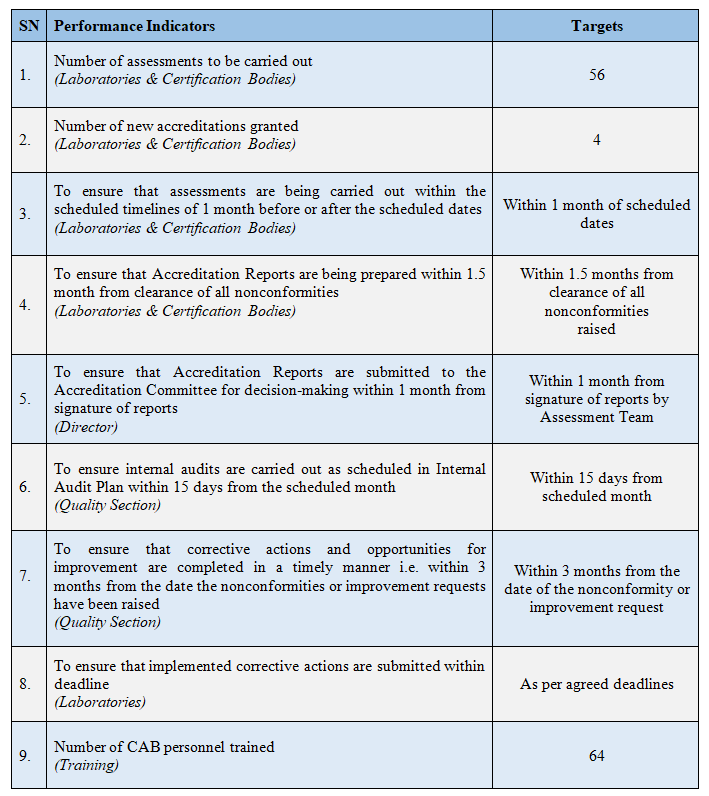
Our Performance Indicators for 2025 - 2026